Sector of Microtechnologies
Sector of Microtechnologies
Collaborations Prof. Allon M. Klein (Harvard Medical School, USA) https://sysbio.med.harvard.edu/facultys/allon-klein-phd Ongoing Projects: H2020-MSCA-ITN-2018, EvoDrops (813786), directed EVOlution in DROPS 01.2.2-MITA-K-702 "Single genome amplification of clinically relevant microorganisms using microfluidics technology", EUREKA Network E! 13634 SAGCHIP 01.2.2-LMT-K-718-04-0002, ”Establishment of single-cell transcriptomics/genomics research parallel-laboratory" 09.3.3O-LMT-K-712-01-0056, "Droplet microfluidics for single-cell genotype phenotype linkage research" |
Linas Mazutis, Ph.D., Prof. Lab Members: Graduate Students: |
Visit our new group's webpage: https://www.gmc.vu.lt/en/sector-of-microtechnologies
Research Overview
Droplet microfluidics technology provides a powerful tool in life sciences. The basic principle of this technology is easy to appreciate: highly monodisperse aqueous droplets are generated in an inert carrier oil in microfluidic channels on a chip and each droplet functions as an independent microreactor. Hence, each droplet is the functional equivalent of a well (or tube), yet the volume of droplet is roughly a thousand to a million times smaller. Such massive reduction in reaction volume provides huge savings in reagents cost when performing large numbers of reactions in parallel. Furthermore, unlike the conventional microtiter plates or valve-based microfluidics, droplets are intrinsically scalable: the number of reaction ‘wells’ is not limited by the physical dimensions of the chip but scales linearly with the emulsion volume. Different microfluidic modules can be employed to manipulate droplets in sophisticated, yet highly controllable manner. Large numbers of droplets (>10^9) can be generated at astonishingly high rates (>20,000 droplets per second), their size tuned precisely, new reagents introduced into pre-formed droplets at defined time points, droplet split and sorted, therefore opening new opportunities for single-cell -omics field. Many useful microfluidic techniques have been developed to profile and even selectively purify single-cells, however, the demand for methods with better analytical performance and improved high-throughput capabilities, remains very high. We are working at fulfilling this demand by bringing higher throughput, reduced reagent cost, scalability and single-molecule resolution for diverse set of quantitative experiments in cell biology and biomedicine.
1. Single-cell barcoding and sequencing using droplet microfluidics
Single-cell sequencing technologies has affected all branches of biological and biomedical sciences, yet one of the major obstacles imposing a real breakthrough is relatively low number of cells that can be isolated and sequenced. Deciphering the unbiased composition of heterogeneous cell populations requires innovative techniques that could capture not hundreds but thousands of single-cells and to do so in a high-throughput manner and affordable cost. This work is built on idea of isolating individual cells into microfluidic droplets carrying RNA/DNA barcodes, and assay reagents. Because individual cells are compartmentalized in drops, cell genetic make up and contents can be accessed. For single-cell studies our approach provides unprecedented scalability, significantly increased throughput, minimal sample loss, reagent savings and large experimental flexibility. Using this technology we are interested in understanding the genetic and epigenetic factors that are responsible for cellular heterogeneity and inheritance.
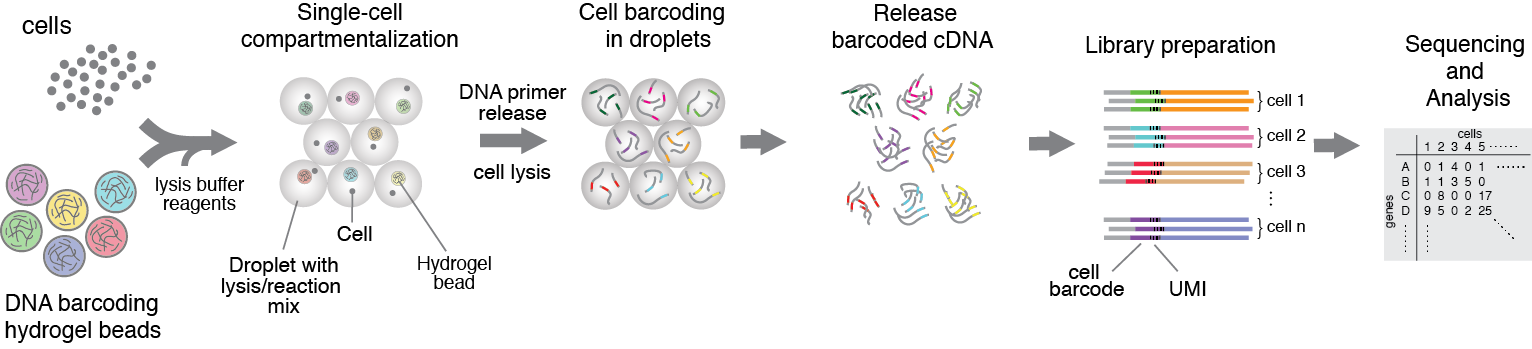
Figure 1. Heterogenious mix of cells is encapsulated into droplets together with reverse-transcription enzyme, lysis mix, and hydrogel beads carrying barcoding primers. After encapsulation cells are lysed, primers are released from the beads and cDNA is tagged with a barcode during reverse transcription reaction. Once cDNA synthesis is complete, droplets are broken and barcoded material from all cells is amplified for next-gen sequencing. Read to learn more
Zilionis R., Nainys J, Veres A., Savova V., Zemmour D., Klein MA., and Mazutis L, (2017) Single-cell barcoding and sequencing using droplet microfluidics, Nature Protocols 12, 44–73
Klein M*, Mazutis L*, Akartuna I*, Tallapragada N, Veres A, Li V, Peshkin L, Weitz D and Kirschner M, (2015) Droplet barcoding for single cell transcriptomics applied to embryonic stem cells, Cell, 161(5): 1187–1201
* - joint first co-authors, http://www.cell.com/cell/first-reflections/mazutis
2. High-throughput screening of antibody secreting cells
In this project, we use droplet microfluidics platform for high-throughput single-cell screening of cells that produce therapeutic antibodies or biomolecules of biomedical interest. Cell compartmentalization into microfluidic droplets together with capture beads and barcoded DNA primers enables us a direct establishment of the linkage between the genotype (genes or mRNA) and phenotype (binding, regulatory or activity of secreted proteins). Due to increased local concentration inside droplets the amount of secreted antibodies will significantly reduce the time window necessary for the assay. We aim at developing a discovery pipeline for the quantitative high-throughput antibody phenotyping without loosing the original heavy-light chain pairing, which would be a significant advantage over other technologies. Our technological approach provides a unique way to identify the primary sequence of heavy and light IgG genes encoding functional monoclonal antibodies directly from single-cells, without a need to perform gene cloning, hybridoma construction or cell immortalization.
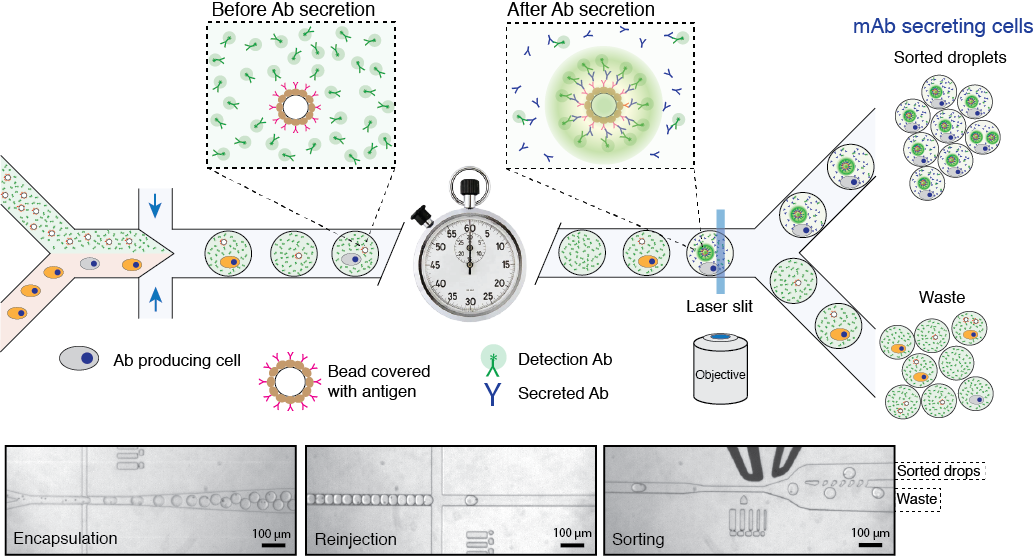
Figure 2. Schematics of microfluidics platform for screening and sequencing single-cells. A mixture of cells is loaded into droplets together with assay reagents and beads carrying capture probe (antigen). The cells secreting antigen specific Ab will be detected by fluorescent sandwich assay on bead surface and sorted. The sorted cells will be lysed and mRNA encoding heavy and light chains are captured and sequenced. Alternatively, the sorted cells can be recovered directly into a growth media for further cultivation, propagation and analysis. Read to learn more
Mazutis L., Gilbert J., Ung L., Weitz D., Griffiths A., Heyman J., (2013) Single-cell analysis and sorting using droplet-based microfluidics, Nature Protocols, 8(5): 870-891.
3. in vitro directed evolution using artificial cells
Darwinian evolution is a powerful algorithm that has given rise to the functionally diverse set of proteins present in all living systems. Repetitive rounds of mutation, selection and amplification have optimized nature’s catalysts, the enzymes, to an extraordinary degree, for example. Biocatalysts are typically much more efficient than their man-made counterparts, often working close to the diffusion limit. A better understanding of how new enzymes evolve consequently remains an important and challenging task for both academic and industrial needs. Although the active sites of natural enzymes are highly complex, making the design of new or promiscuous protein functions difficult, computation has recently emerged as a potentially powerful strategy for creating protein catalysts with tailored activities and specificities. Because the activities of such artificial enzymes are still modest, we plan to explore the feasibility of optimizing them by combining the latest advances in droplet-based microfluidics technology with the methods of directed evolution. Specifically, two complimentary groups, one with expertise in microfluidic systems and another with expertise in directed protein evolution, will join forcess to investigate mechanisms and strategies for optimizing several computationally designed esterases. By applying droplet-based microfluidics technology, in which biochemical reactions are performed inside micrometer size vessels, we will have a powerful tool enabling large number of libraries (>10^6) to be screened at ultra-high-throughput rates and using conditions that are incompatible with in vivo systems.
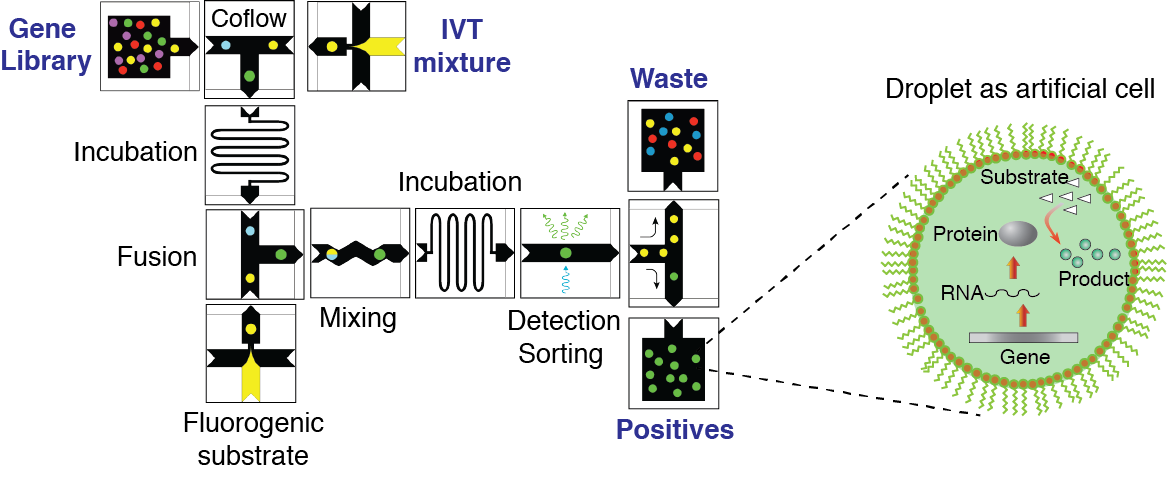
Figure 3. Concept of directed evolution approach in droplet microfluidics. Droplets containing single-genes with all ingredients necessary for in vitro expression will serve as artificial cells that can be selected for a desirable phenotype under conditions that are not feasible in living systems. Library of mutated genes is encapsulated such that, statistically, the majority of droplets contain no more than one gene per droplet. After IVTT reaction the droplets are fused with a second type of droplet containing a fluorogenic substrate and other molecules, salts, and reagents needed to impose the selection pressure. After mixing and incubation, the fluorescence of each droplet is recorded. Droplets with fluorescence above a threshold are sorted by means of dielectrophoresis and the genes contained therein are amplified using PCR. The selected genes can be either characterized, re-selected, or mutated for subsequent round of directed evolution.
Galinis R, Stonyte G, Kiseliovas V, Zilionis R, Studer S, Hilvert D, Janulaitis A and Mazutis L. (2016) DNA nanoparticles for improved protein synthesis in vitro, Angew Chem Int Ed Engl. 2016 Feb 24;55(9):3120-3
4. Microfluidic tools for biological and biomedical applications
Our group has long history of experience at developing novel microfluidic tools for precise manipulation and analysis of biological reactions at pico and nanoliter range volumes. We provide designs, modeling, fabrication and characterization of microfluidic systems and devices for myriad of biological and biomedical applications. We build experimental platforms based on integration of microfluidics, mechanical, electrical and optical modules eventually enabling precise control over experimental conditions hardly feasible in bulk. Using droplet microfluidic devices we provide significantly reduced assay volumes for virtually any biological assay and as a result big cost savings for biochemical reagents and compounds. We are developing microfluidic devices and chips for digital DNA/RNA analysis, single-cell and genomic applications. In addition to droplet microfluidic systems in collaboration with Dr. Jonathan Thon and Prof. Joseph Italiano (Brigham and Women's Hospital, USA) we have developed a micro-bioreactor for generation of synthetic human platelets in vitro. This work formed a technological base for establishment of a start-up company Platelet Biogenesis Inc., which is based in Boston, MA.
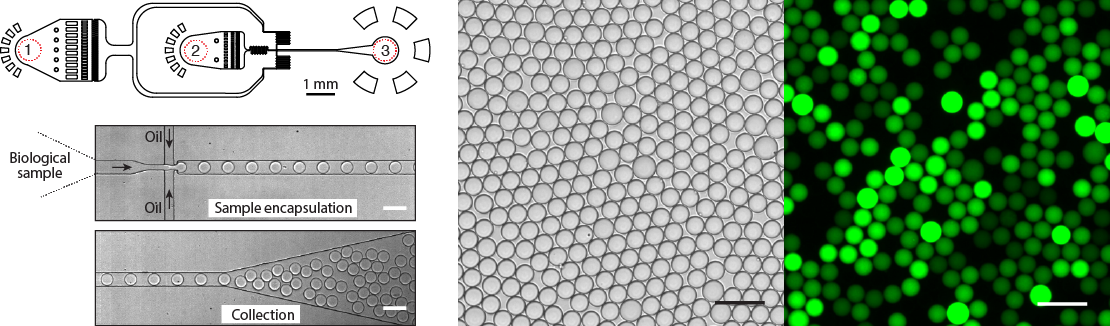
Figure 4. Design and operation of the droplet microfluidics device. Almost any biological sample (DNA, cells, proteins) can be encapsulated into pico- or nano-liter volume droplets at >10.000 droplets per second. Typical microfluidics device has (1) the inlet for the continuous phase, (2) the inlet for biological sample, and (3) the droplet collection outlet. Bright field and fluorescence images of an emulsion after DNA amplification reaction. Droplets containing amplified DNA exhibit green fluorescence, whereas droplets lacking a template are dark. Scale bars, 50 um.
Mazutis L, Araghi AF, Miller OJ, Baret JC, Frenz L, Janoshazi A, Taly V, Miller BJ, Hutchison JB, Link D, Griffiths AD, Ryckelynck M., (2009) Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis, Analytical Chemistry 81(12): 4813-21.
Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, Feng Q, Lu S, Lanza R, Neeves KB, Weitz DA, Italiano JE (2014) Platelet bioreactor-on-a-chip, Blood, 124(12): 1857-1867
Mazutis L. and A. Griffiths (2012) “Selective droplet coalescence using microfluidic systems”. Lab on a Chip, 12, 1800-1806.
5. Drug delivery systems
Vesicles and microspheres composed of biodegradable polymers are becoming increasingly attractive agents for improved delivery, stabilization and prolonged release of drugs. However, the vast majority of techniques being used for microspheres production are based on mechanical steering or sonication. These methods, although efficient, produce particles with large polydispersity (coefficient of variance C.V. ~10-30%) and therefore impose several limitations. Particle’s size distribution may produce uncontrolled variation in the rate of particle degradation, affect the kinetics of drug release and decrease the stability of encapsulated drugs. In contrast, monodisperse drug-loaded particles offer significantly better control over release kinetics and exhibit a much lower initial drug release burst that is observed when using polydisperse particles. We aim to develop droplet-based microfluidics technology that will be used to create highly monodisperse (C.V. < 0.5%) vesicles and particles for improved drug encapsulation, delivery and release. Microfluidic systems offer a novel way to create and manipulate fluids that is hardly possible using conventional techniques. For instance, miniaturized microfluidic systems have been used to create polymer particles composed of thermosensitive, mechanosensitive, ion-sensitive and biodegradable materials, finding many useful applications in biotechnology and chemical industry. In this project we will develop and apply microfluidic technology for production of nano- and pico-liter volume particles in which inner part of the vesicle will contain preloaded biological or chemical molecules and outer part (shell) will be composed of biodegradable polymer. Additional advantage of using microfluidics is that inner and outer parts of the vesicles can be tuned by controlling the volumetric flow rate ratio, thus allowing precise control over the vesicle size and thickness of the shell.
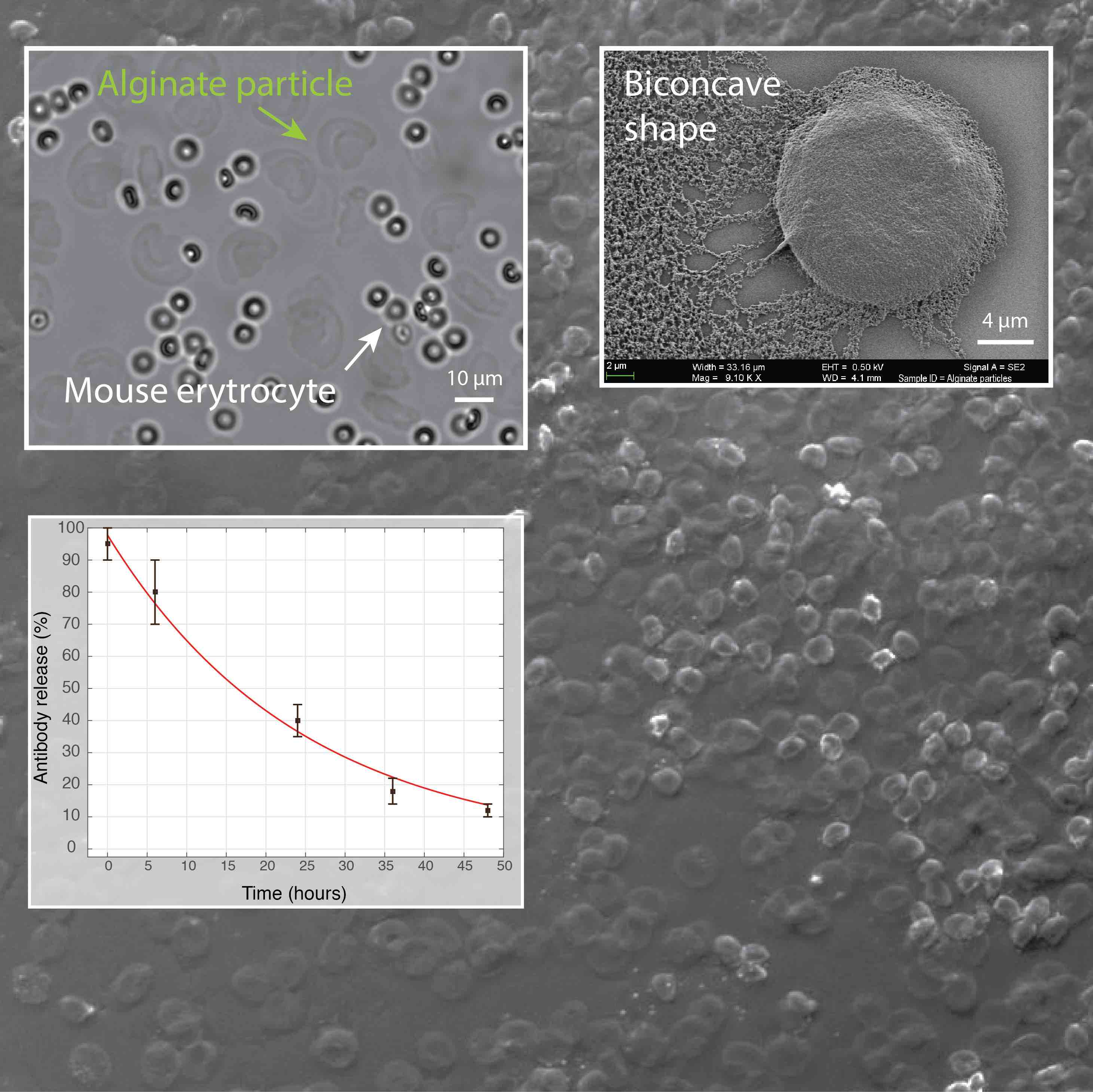
Figure 5. We have developed a microfluidic approach for production of hydrogel particles having the unique biconcave shape and the size of a mammalian cell (~10 µm). The release of encapsulated antibodies was largely affected by the presence of phosphate ions that chelate calcium (a cross-linker of alginate polymer) and cause the dissolution of hydrogel. In addition, the image shows the biocompatibility of alginate particles in the whole blood.
Vasiliauskas R, Liu D, Cito S, Zhang H, Shahbazi MA, Sikanen T, Mazutis L, Santos HA (2015) A Simple Microfluidic Approach to Fabricate Monodisperse Hollow Microparticles for Multidrug Delivery, ACS Appl. Mater. Interfaces, 7 (27), pp 14822–14832.
Mazutis L, Vasiliauskas R, Weitz D, (2015) Microfluidic Production of Alginate Hydrogel Particles for Antibody Encapsulation and Release, Macromol. Biosci. 2015, 15, 1641–1646
6. DNA-Magnesium-pyrophosphate particle biosynthesis and their use
Compartmentalization and amplification of single DNA molecules inside nano- or pico-liter sized wells and droplets opens new opportunities for biomedical and biological sciences. The most common method of amplifying DNA in a sample involves the polymerase chain reaction (PCR). Yet, amplification of long (>1 kb) single-molecule templates is often inefficient, leading to reduced reaction yields and biases. DNA amplification under isothermal reaction conditions has been shown to generate large amounts of material from a single-copy DNA template. Moreover, the ability to amplify DNA and then express proteins from the clonally amplified template would greatly increase the scope of potential applications.
We employ droplet microfluidics devices to encapsulate and convert single DNA molecules into condensed DNA nanoparticles by a multiple displacement amplification (MDA) reaction driven by phi29 DNA polymerase. The condensed DNA nanoparticles comprise up to ~10.000-100.000 copies of clonally amplified DNA template. Intriguingly, we found that inorganic pyrophosphate, produced during isothermal DNA synthesis, and magnesium ions are a prerequisite for DNA condensation into the crystalline-like globular structures. This process is enhanced when the DNA amplification reaction is performed inside droplets, which can be attributed to the confined volumes and spatial accumulation of the reaction products. We have demonstrated the biological functionality of the DNA nanoparticles, by applying them in in vitro transcription-translation reactions and observed improved protein expression yields relative to standard assay conditions.
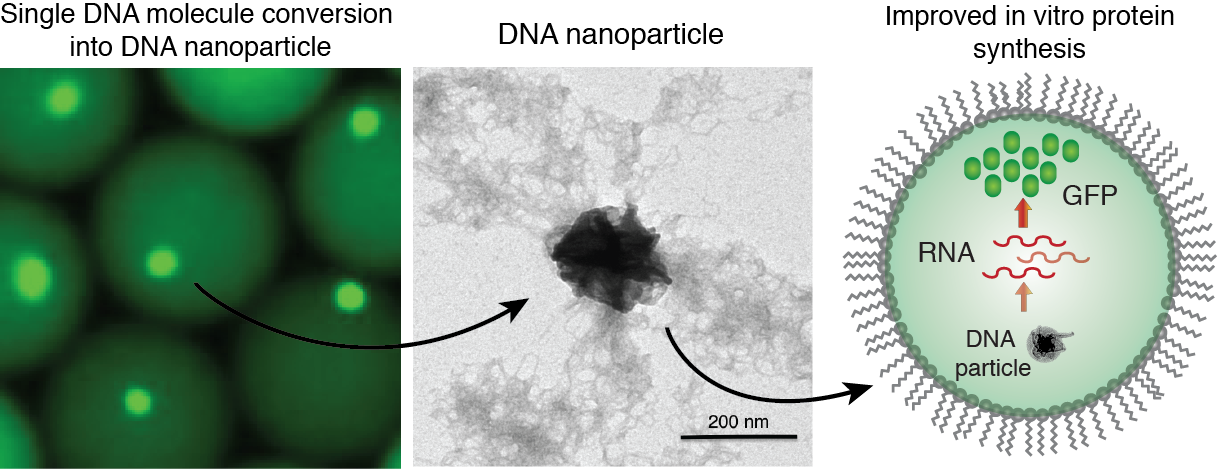
Figure 6. Generation of DNA-Mg-PP particles. DNA nanoparticle formation induced by inorganic pyrophosphate (PP) and magnesium ions during a phi29-catalyzed DNA polymerization reaction. TEM image of the DNA-Mg-PP particle. The use of DNA for efficient gene expression and protein synthesis in vitro. Read to learn more
Galinis R, Stonyte G, Kiseliovas V, Zilionis R, Studer S, Hilvert D, Janulaitis A and Mazutis L. (2016) DNA nanoparticles for improved protein synthesis in vitro, Angew Chem Int Ed Engl. 2016 Feb 24;55(9):3120-3
Zubaite, G.; Simutis, K.; Galinis, R.; Milkus, V.; Kiseliovas, V.; Mazutis, L. Droplet Microfluidics Approach for Single-DNA Molecule Amplification and Condensation into DNA-Magnesium-Pyrophosphate Particles. Micromachines 2017, 8, 62.
Funding sources
European Commission, H2020 program http://ec.europa.eu/research/mariecurieactions/
Lithuanian-Swiss Research and Development cooperation program https://www.cpva.lt/en/lithuanian-swiss-cooperation-programme
The Scientific Exchange Programme (Sciex-NMSch) https://www.swissuniversities.ch/en/topics/sciex/
Agency for Science, Innovation and Technology (MITA) http://www.mita.lt/en
Lithuanian Research Council http://www.lmt.lt/en/about.html
Important publications of our group:
1. Klein M*, Mazutis L*, Akartuna I*, Tallapragada N, Veres A, Li V, Peshkin L, Weitz D and Kirschner M, (2015) Droplet barcoding for single cell transcriptomics applied to embryonic stem cells, Cell, 161(5): 1187–1201
2. Zilionis R., Nainys J, Veres A., Savova V., Zemmour D., Klein MA., and Mazutis L, (2017) Single-cell barcoding and sequencing using droplet microfluidics, Nature Protocols 12, 44–73
3. Azizi E, Carr A, Plitas G., Cornish EA, Konopacki C, Prabhakaran S, Nainys J., Wu K. Kiseliovas V., Setty M., Choi K., Dao P, Mazutis L, Rudensky YA & Peer D, Single-cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment (2018), Cell 174, 1–16, August 23
4. Setty M, Kiseliovas V, Levine J, Gayoso A, Mazutis L, Pe'er D., (2019) Characterization of cell fate probabilities in single-cell data with Palantir, Nat Biotechnol. 2019 Apr;37(4):451-460.
5. Chi, Y., J. Remsik, V. Kiseliovas, C. Derderian, U. Sener, M. Alghader, F. Saadeh, K. Nikishina, T. Bale, C. Iacobuzio-Donahue, T. Thomas, D. Pe'er, L. Mazutis and A. Boire (2020). "Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis." Science 369(6501): 276-282.
Hot publications:
1. Pritykin, Y., J. van der Veeken, A. R. Pine, Y. Zhong, M. Sahin, L. Mazutis, D. Pe'er, A. Y. Rudensky and C. S. Leslie (2021). "A unified atlas of CD8 T cell dysfunctional states in cancer and infection." Mol Cell 3;81(11):2477-2493
2. Alonso-Curbelo, D., Y. J. Ho, C. Burdziak, J. L. V. Maag, J. P. t. Morris, R. Chandwani, H. A. Chen, K. M. Tsanov, F. M. Barriga, W. Luan, N. Tasdemir, G. Livshits, E. Azizi, J. Chun, J. E. Wilkinson, L. Mazutis, S. D. Leach, R. Koche, D. Pe'er and S. W. Lowe (2021). "A gene-environment-induced epigenetic program initiates tumorigenesis." Nature 590(7847): 642-648.
3. Leonaviciene, G., Leonavicius, K., Meskys, R. & Mazutis, L. Multi-step processing of single cells using semi-permeable capsules. Lab on a Chip 20, 4052-4062 (2020).
Publications:
2017-present
Pritykin, Y., J. van der Veeken, A. R. Pine, Y. Zhong, M. Sahin, L. Mazutis, D. Pe'er, A. Y. Rudensky and C. S. Leslie (2021). "A unified atlas of CD8 T cell dysfunctional states in cancer and infection." Mol Cell 3;81(11):2477-2493
Guttikonda, S. R., L. Sikkema, J. Tchieu, N. Saurat, R. M. Walsh, O. Harschnitz, G. Ciceri, M. Sneeboer, L. Mazutis, M. Setty, P. Zumbo, D. Betel, L. D. de Witte, D. Pe'er and L. Studer (2021). "Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer's disease." Nat Neurosci 24(3): 343-354.
Alonso-Curbelo, D., Y. J. Ho, C. Burdziak, J. L. V. Maag, J. P. t. Morris, R. Chandwani, H. A. Chen, K. M. Tsanov, F. M. Barriga, W. Luan, N. Tasdemir, G. Livshits, E. Azizi, J. Chun, J. E. Wilkinson, L. Mazutis, S. D. Leach, R. Koche, D. Pe'er and S. W. Lowe (2021). "A gene-environment-induced epigenetic program initiates tumorigenesis." Nature 590(7847): 642-648.
2020
The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Rozenblatt-Rosen O, Regev A, Oberdoerffer P, Nawy T, Hupalowska A, Rood JE, Ashenberg O, Cerami E, Coffey RJ, Demir E, Ding L, Esplin ED, Ford JM, Goecks J, Ghosh S, Gray JW, Guinney J, Hanlon SE, Hughes SK, Hwang ES, Iacobuzio-Donahue CA, Jané-Valbuena J, Johnson BE, Lau KS, Lively T, Mazzilli SA, Pe'er D, Santagata S, Shalek AK, Schapiro D, Snyder MP, Sorger PK, Spira AE, Srivastava S, Tan K, West RB, Williams EH; Human Tumor Atlas Network. Cell. 2020 Apr 16;181(2):236-249.
Laughney AM, Hu J, Campbell NR, Bakhoum SF, Setty M, Lavallée VP, Xie Y, Masilionis I, Carr AJ, Kottapalli S, Allaj V, Mattar M, Rekhtman N, Xavier JB, Mazutis L, Poirier JT, Rudin CM, Pe'er D, Massagué J. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med. 2020 Feb;26(2):259-269.
Leonaviciene, G., K. Leonavicius, R. Meskys and L. Mazutis (2020). "Multi-step processing of single cells using semi-permeable capsules." Lab Chip 20(21): 4052-4062.
Gegevicius, E., Goda, K., and Mazutis, L. (2020). CHAPTER 4:Droplet Gene Analysis – Digital PCR. In Droplet Microfluidics, pp. 89–121. DOI: 10.1039/9781839162855-00089
Chi, Y., J. Remsik, V. Kiseliovas, C. Derderian, U. Sener, M. Alghader, F. Saadeh, K. Nikishina, T. Bale, C. Iacobuzio-Donahue, T. Thomas, D. Pe'er, L. Mazutis and A. Boire (2020). "Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis." Science 369(6501): 276-282.
Ding, R. H., K. C. Hung, A. Mitra, L. W. Ung, D. Lightwood, R. Tu, D. Starkie, L. H. Cai, L. Mazutis, S. R. Chong, D. A. Weitz and J. A. Heyman (2020). "Rapid isolation of antigen-specific B-cells using droplet microfluidics." RSC Advances 10(45): 27006-27013.
Karthaus, W. R., M. Hofree, D. Choi, E. L. Linton, M. Turkekul, A. Bejnood, B. Carver, A. Gopalan, W. Abida, V. Laudone, M. Biton, O. Chaudhary, T. H. Xu, I. Masilionis, K. Manova, L. Mazutis, D. Pe'er, A. Regev and C. L. Sawyers (2020). "Regenerative potential of prostate luminal cells revealed by single-cell analysis." Science 368(6490): 497.
Kim, S. H., A. Holland, A. Jimenez-Sanchez, Y. Bykov, R. M. Fromme, A. Stylianou, T. Walther, C. Liu, M. M. Leitao, O. Zivanovic, Y. Sonoda, D. S. Chi, N. R. Abu-Rustum, L. Mazutis, G. Plitas, T. J. Hollmann, B. Weigelt, D. Pe'er and D. Zamarin (2020). "Compositional and architectural characterization of high-grade serous ovarian carcinomas using single cell technologies and multiplex microscopy." Gynecologic Oncology 159: 44-44.
Marjanovic, N. D., M. Hofree, J. E. Chan, D. Canner, K. Wu, M. Trakala, G. G. Hartmann, O. C. Smith, J. Y. Kim, K. V. Evans, A. Hudson, O. Ashenberg, C. B. M. Porter, A. Bejnood, A. Subramanian, K. Pitter, Y. Yan, T. Delorey, D. R. Phillips, N. Shah, O. Chaudhary, A. Tsankov, T. Hollmann, N. Rekhtman, P. P. Massion, J. T. Poirier, L. Mazutis, R. Li, J. H. Lee, A. Amon, C. M. Rudin, T. Jacks, A. Regev and T. Tammela (2020). "Emergence of a High-Plasticity Cell State during Lung Cancer Evolution." Cancer Cell 38(2): 229-246 e213.
Morello, F., D. Borshagovski, M. Survila, L. Tikker, S. Sadik-Ogli, A. Kirjavainen, N. Estartus, L. Knaapi, L. Lahti, P. Toronen, L. Mazutis, A. Delogu, M. Salminen, K. Achim and J. Partanen (2020). "Molecular Fingerprint and Developmental Regulation of the Tegmental GABAergic and Glutamatergic Neurons Derived from the Anterior Hindbrain." Cell Rep 33(2): 108268.
Xue, J. Y., Y. L. Zhao, J. Aronowitz, T. T. Mai, A. Vides, B. Qeriqi, D. Kim, C. C. Li, E. de Stanchina, L. Mazutis, D. Risso and P. Lito (2020). "Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition." Nature 577(7790): 421-425.
2019
Cai XC, Zhang T, Kim EJ, Jiang M, Wang K, Wang J, Chen S, Zhang N, Wu H, Li F, Dela Seña CC, Zeng H, Vivcharuk V, Niu X, Zheng W, Lee JP, Chen Y, Barsyte D, Szewczyk M, Hajian T, Ibáñez G, Dong A, Dombrovski L, Zhang Z, Deng H, Min J, Arrowsmith CH, Mazutis L, Shi L, Vedadi M, Brown PJ, Xiang J, Qin LX, Xu W, Luo M. A chemical probe of CARM1 alters epigenetic plasticity against breast cancer cell invasion. eLife. 2019 Oct 28;8:e47110.
Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée VP, Mendoza A, Fromme R, Mazutis L, Ariyan C, Leslie C, Pe'er D, Rudensky AY. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell. 2019 Oct 31;179(4):846-863.e24.
Setty M, Kiseliovas V, Levine J, Gayoso A, Mazutis L, Pe'er D., (2019) Characterization of cell fate probabilities in single-cell data with Palantir, Nat Biotechnol. 2019 Apr;37(4):451-460.
van der Veeken J, Zhong Y, Sharma R, Mazutis L, Dao P, Pe'er D, Leslie CS, Rudensky AY. (2019) Natural Genetic Variation Reveals Key Features of Epigenetic and Transcriptional Memory in Virus-Specific CD8 T Cells, Immunity. 2019 May 21;50(5):1202-1217
Nainys, Juozas, Valdemaras Milkus, and Linas Mažutis. "Single-cell screening using microfluidic systems." Microfluidics for Pharmaceutical Applications. William Andrew Publishing, 2019. 353-367.
Karolis S, Stonyte G, and Mažutis L. "Antibody discovery using microfluidic systems." Microfluidics for Pharmaceutical Applications. William Andrew Publishing, 2019. 337-351.
2018
X Fan, B Moltedo, A Mendoza, AN Davydov, MB Faire, L Mazutis, R Sharma, D Pe’er, DM Chudakov, AY Rudensky, CD49b defines functionally mature Treg cells that survey skin and vascular tissues (2018), Journal of Experimental Medicine 215 (11), 2796-2814
K Leonavicius, J Nainys, D Kuciauskas, L Mazutis, Multi-omics at single-cell resolution: comparison of experimental and data fusion approaches, (2019) Current Opinion in Biotechnology 55, 159-166
Azizi E, Carr A, Plitas G., Cornish EA, Konopacki C, Prabhakaran S, Nainys J., Wu K. Kiseliovas V., Setty M., Choi K., Dao P, Mazutis L, Rudensky YA & Peer D, Single-cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment (2018), Cell 174, 1–16, August 23
van Dijk D, Sharma R, Nainys J, Yim K, Kathail P, Carr A., Burdziak C., Moon RK,Chaffer C., Pattabiraman D, Bierie B, Mazutis L., Wolf G., Krishnaswamy S., Peer D.
Recovering gene interactions from single-cell data using data diffusion, (2018) Cell, 174, 716-729, July 29
2010-2017
Zilionis R., Nainys J, Veres A., Savova V., Zemmour D., Klein MA., and Mazutis L, (2017) Single-cell barcoding and sequencing using droplet microfluidics, Nature Protocols 12, 44–73
Einav T., Mazutis L, and Phillips R, (2016) Statistical Mechanics of Allosteric Enzymes, J. Phys. Chem. B, 120 (26), pp 6021–6037.
Galinis R, Stonyte G, Kiseliovas V, Zilionis R, Studer S, Hilvert D, Janulaitis A and Mazutis L. (2016) DNA nanoparticles for improved protein synthesis in vitro, Angew. Chem. Int Ed, 55, 3120-3123
Wagner O, Thiele J, Weinhart M, Mazutis L, Weitz D, Huck W Haag R. (2016) Biocompatible fluorinated polyglycerols for droplet microfluidics as an alternative to PEG- based copolymer surfactants, Lab on a Chip, 16, 65-69
Mazutis L, Vasiliauskas R, Weitz D, (2015) Microfluidic Production of Alginate Hydrogel Particles for Antibody Encapsulation and Release, Macromol. Biosciences, 15, 1641–1646
Vasiliauskas R, Liu D, Cito S, Zhang H, Shahbazi MA, Sikanen T, Mazutis L, Santos HA (2015) A Simple Microfluidic Approach to Fabricate Monodisperse Hollow Microparticles for Multidrug Delivery, ACS Appl. Mater. Interfaces, 7 (27), pp 14822–14832.
Bender M, Thon JN, Ehrlicher AJ, Wu S, Mazutis L, Deschmann E, Sola-Visner M, Italiano JE Jr, Hartwig JH. (2015) Dynein-dependent microtubule sliding drives proplatelet elongation, Journal of Thrombosis and Haemostasis, 13:184.
Klein M*, Mazutis L*, Akartuna I*, Tallapragada N, Veres A, Li V, Peshkin L, Weitz D and Kirschner M, (2015) Droplet barcoding for single cell transcriptomics applied to embryonic stem cells, Cell, 161(5): 1187–1201 * - joint first co-authors,
Bender M, Thon JN, Ehrlicher AJ, Wu S, Mazutis L, Deschmann E, Sola-Visner M, Italiano JE Jr, Hartwig JH. (2015) Microtubule sliding drives proplatelet elongation and is dependent on cytoplasmic dynein, Blood, 125(5): 860-868
Shekhar S., L. Zhu, L. Mazutis, A. E. Sgro, T. G. Fai, and M. Podolski (2014) Quantitative biology: where modern biology meets physical sciences, Molecular Biology of the Cell, Nov.5, vol.25, no.22, 3482.
Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, Feng Q, Lu S, Lanza R, Neeves KB, Weitz DA, Italiano JE (2014) Platelet bioreactor-on-a-chip, Blood, 124(12): 1857-1867
Mazutis L., Gilbert J., Ung L., Weitz D., Griffiths A., Heyman J., (2013) Single-cell analysis and sorting using droplet-based microfluidics, Nature Protocols, 8(5): 870-891
Mazutis L. and A. Griffiths (2012) Selective droplet coalescence using microfluidic systems, Lab on a Chip, 12, 1800-1806.
Skhiri, Y.; Gruner, P.; Semin, B.; Brosseau, Q.; Pekin, D.; Mazutis, L.; Goust, V.; Kleinschmidt, F.; El Harrak, A.; Hutchison, J. B.; Mayot, E.; Bartolo, J. F.; Griffiths, A. D.; Taly, V.; Baret, J. C., (2012) Dynamics of molecular transport by surfactants in emulsions, Soft Matter, 8 (41), 10618-10627.
Pekin, D.; Skhiri, Y.; Baret, J. C.; Le Corre, D.; Mazutis, L.; Salem, C. B.; Millot, F.; El Harrak, A.; Hutchison, J. B.; Larson, J. W.; Link, D. R.; Laurent-Puig, P.; Griffiths, A. D.; Taly, V., (2011) Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip, 11(13), 2156-66.
Until 2010
Mazutis L. and A. D. Griffiths (2009) Preparation of monodisperse emulsions by hydrodynamic size fractionation, Appl. Phys. Lett. 95(20): 204103.
Mazutis L, Baret JC, Treacy P, Skhiri Y, Araghi AF, Ryckelynck M, Taly V, Griffiths AD. (2009) Multi-step microfluidic droplet processing: kinetic analysis of an in vitro translated enzyme, Lab on a Chip 9(20): 2902-8.
Mazutis L, Baret JC, Griffiths AD (2009) A fast and efficient microfluidic system for highly selective one-to-one droplet fusion, Lab Chip 9(18): 2665-72
Mazutis L, Araghi AF, Miller OJ, Baret JC, Frenz L, Janoshazi A, Taly V, Miller BJ, Hutchison JB, Link D, Griffiths AD, Ryckelynck M. (2009) Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis, Analytical Chemistry 81(12): 4813-21.






